Stainless steel is classified into several types based on its microstructure, significantly influencing its properties and applications. There are five main types of stainless steel:
1. Ferritic – These steels are based on Chromium with small amounts of Carbon, usually less than 0.10%. These steels have a microstructure similar to Carbon and low alloy steels. They are usually limited to relatively thin sections due to the lack of toughness in welds. However, where welding is not required, they offer a wide range of applications. They cannot be hardened by heat treatment. High Chromium steels with additions of Molybdenum can be used in aggressive conditions such as seawater. Ferritic steels are also chosen for their resistance to stress corrosion cracking. They are not as formable as austenitic stainless steels. They are magnetic.
FERRITIC STAINLESS STEELS
Introduction
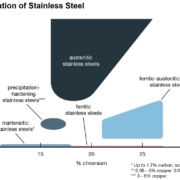
Stainless steel is the name given to a family of corrosion and heat-resistant steels containing a minimum of 10.5% chromium. Just as there is a range of structural and engineering carbon steels meeting different requirements of strength, weldability, and toughness, so there is a wide range of stainless steels with progressively higher corrosion resistance and strength levels. This results from the controlled addition of alloying elements, each offering specific attributes regarding strength and ability to resist different environments. The available grades of stainless steel can be classified into five basic families: ferritic, martensitic, austenitic, duplex, and precipitation hardenable.
Ferritic stainless steels
Ferritic stainless steels have a “Body-Centered-Cubic” (BCC) crystal structure, the same as pure iron at room temperature.

The main alloying element is chromium, with contents typically between 11 and 17%, although a higher chromium content of about 29% is found in one specialized grade. Carbon is kept low, which results in these steels having limited strength. They are not hardenable by heat treatment and have annealed yield strengths in the 275 to 350 MPa range.
Ferritics are usually lower in cost than austenitic steel due to the absence of nickel. They have often been thought of as also having lower corrosion resistance. However, stabilized grades such as 1.4509 and 1.4521 are similar in corrosion resistance to 1.4301 (304) and 1.4401 (316). The main disadvantages of the ferritic are:
- Limited toughness – Not acceptable for sub-zero temperatures
- Formability – Good for deep drawing, but not stretch forming due to lower ductility
- Weldability – Rapid grain growth in thick sections (greater than 3mm) leads to poor weld toughness compared to the austenitic.
2. Austenitic – These steels are the most common. Their microstructure is derived from the addition of Nickel, Manganese, and Nitrogen. It has the same structure as ordinary steel at much higher temperatures. This structure gives these steels their characteristic combination of weldability and formability. Corrosion resistance can be enhanced by adding Chromium, Molybdenum, and Nitrogen. They cannot be hardened by heat treatment but have the valuable property of being work-hardened to high strength levels while retaining proper flexibility and toughness. Standard austenitic steels are vulnerable to stress corrosion cracking. Higher nickel austenitic steels have increased resistance to stress corrosion cracking. They are nominally non-magnetic but usually exhibit some magnetic response depending on the steel’s composition and work hardening.
AUSTENITIC STAINLESS STEELS
Introduction
Stainless steel is the name given to a family of corrosion and heat-resistant steels containing a minimum of 10.5% chromium. Just as a range of structural and engineering carbon steels meet different requirements of strength, weldability, and toughness, there is a wide range of stainless steels with progressively higher corrosion resistance and strength levels. This results from the controlled addition of alloying elements, each offering specific attributes regarding strength and ability to resist different environments. The available grades of stainless steel can be classified into five basic families: ferritic, martensitic, austenitic, duplex, and precipitation hardenable.
Austenitic stainless steels
Austenitic stainless steels have a “Face-Centered-Cubic” (FCC) crystal structure, the same as pure iron above the A3 temperature of 910°C. Pure iron maintains this structure until it reaches the A4 temperature of 1390 °C and then reverts to the BCC structure.


This type of stainless steel has two main alloying elements, i.e., Chromium and Nickel. The classic austenitic stainless steel is the “18:8” alloy, which contains 18% Chromium and 8% Nickel. These alloys mainly rely on Nickel additions to stabilize the Austenitic/FCC structure, but other elements such as Manganese, Copper, and Nitrogen also exhibit this property of stabilizing the Austenitic structure. These steels are not heat-treatable, so they can not be hardened in this manner; however, depending upon their exact composition, they can show very high levels of work hardening.
Because of their face-centered cubic structure, they are highly ductile and can be readily formed into various shapes.
Their composition can be altered to maximize formability for deep drawing or stretch forming.
Their face-centered cubic structure also means they are non-magnetic, but specific compositions within this group can become weakly magnetic, especially after cold working. However, it is also possible to manipulate their composition so they do not become even slightly magnetic after such work.
Austenitic stainless steels generally exhibit good weldability and toughness even down to cryogenic temperatures; they do not display a ductile to brittle transition with decreasing temperature.
3. Martensitic—These steels are similar to ferritic steels based on Chromium but have higher Carbon levels, up to 1%. This makes them hardened and tempered, much like Carbon and low-alloy steels. They are used where high strength and moderate corrosion resistance are required. They are more common in long products than in sheet and plate form. They generally have low weldability and formability. They are magnetic.
MARTENSITIC STAINLESS STEELS
Introduction
Stainless steel is the name given to a family of corrosion and heat-resistant steels containing a minimum of 10.5% chromium. Just as there is a range of structural and engineering carbon steels meeting different requirements of strength, weldability, and toughness, so there is a wide range of stainless steels with progressively higher corrosion resistance and strength levels. This results from the controlled addition of alloying elements, each offering specific attributes regarding strength and ability to resist different environments. The available grades of stainless steel can be classified into five basic families: ferritic, martensitic, austenitic, duplex, and precipitation hardenable.
Martensitic Stainless Steels
Martensitic stainless steels are similar to low alloy or carbon steels. In the annealed condition, they have a structure similar to the ferritic, but when hardened, they have a ‘body-centered tetragonal’ (but) crystal lattice rather than a body-centered cubic (bcc) lattice. Due to the deliberate addition of carbon, they can be hardened and strengthened by heat treatment, similar to many carbon/carbon alloy steels. They are classed as a “hard” ferromagnetic group. The main alloying element is chromium, with a typical 12-15% content.
In the annealed condition (where they do have a body-centered cubic (bcc) lattice structure), they have tensile yield strengths of about 275 MPa. So, they are usually machined, cold-formed, or cold-worked in this condition. The strength obtained by heat treatment depends on the carbon content of the alloy. Increasing the carbon content increases the strength and hardness potential but decreases ductility and toughness. The higher carbon grades can be heat treated to hardnesses of 60 HRC.
Optimum corrosion resistance is attained in heat-treated conditions, i.e., hardened and tempered. Tempering after hardening, whilst softening the steels somewhat, also restores a degree of ductility. Martensitic grades have been developed with nitrogen and nickel additions but with lower carbon levels than the traditional grades. These steels have improved toughness, weldability, and corrosion resistance.
Examples of martensitic grades are 420S45 (1.4028) and 431 (1.4057), which are traditional carbon hardenable grades, and 248SV (1.4418), which is one of the low carbon/nitrogen grades.
4. Duplex – These steels have a microstructure of approximately 50% ferritic and 50% austenitic. This gives them a higher strength than either ferritic or austenitic steels. They are resistant to stress corrosion cracking. So-called “lean duplex” steels are formulated to have comparable corrosion resistance to standard austenitic steels but with enhanced strength and resistance to stress corrosion cracking. “Superduplex” steels have enhanced strength and resistance to all forms of corrosion compared to standard austenitic steels. They are weldable but need care when selecting welding consumables and heat input. They have moderate formability. They are magnetic, but not so much as the ferritic, martensitic, and PH grades due to the 50% austenitic phase.
DUPLEX STAINLESS STEELS
Duplex Stainless Steels
Duplex stainless steels are becoming more common. They are being offered by all the major stainless steel mills for several reasons:
- Higher strength leads to weight-saving
- More excellent corrosion resistance, particularly to stress corrosion cracking
- Better price stability
- Lower price
Every 2-3 years, a conference on duplex is held, at which dozens of highly technical papers are presented. There is a lot of marketing activity surrounding these grades, and new grades are frequently announced.
Yet, even with all this interest, the best estimates for global market share for duplex are between 1 and 3%. This article aims to provide a straightforward guide to this steel type. The advantages and disadvantages will be described.
Principle of Duplex Stainless Steels
The idea of duplex stainless steel dates back to the 1920s, with the first cast being made at Avesta in Sweden in 1930. However, it is only in the last 30 years that duplex steels have begun to “take off” significantly. This is mainly due to advances in steelmaking techniques, particularly concerning the control of nitrogen content.
The standard austenitic steels like 304 (1.4301) and ferritic steels like 430 (1.4016) are relatively easy to make and fabricate. As their names imply, they consist mainly of one phase: austenite or ferrite. Although these types are fine for a wide range of applications, there are some important technical weaknesses in both types:
Austenitic – low strength (200 MPa 0.2% PS in solution annealed condition), low resistance to stress corrosion cracking
Ferritic – low strength (a bit higher than austenitic, 250 MPa 0.2% PS), poor weldability in thick sections, poor low-temperature toughness
In addition, the high nickel content of the austenitic types leads to price volatility, which is unwelcome to many end users.
The basic idea of duplex is to produce a chemical composition that leads to an approximately equal mixture of ferrite and austenite. This balance of phases provides the following:
- Higher strength – The range of 0.2% PS for the current duplex grades is from 400 – 550 MPa. This can lead to reduced section thicknesses and, therefore, to reduced weight. This advantage is particularly significant for applications such as:
o Pressure Vessels and Storage Tanks
o Structural Applications, e.g., bridges
- Good weldability in thick sections – Not as straightforward as austenitic, but much better than ferritic.
- Good toughness—Much better than ferritics, particularly at low temperatures, typically down to minus 50 deg. C, stretching to minus 80 deg. C.
- Resistance to stress corrosion cracking – Standard austenitic steels are particularly prone to this type of corrosion. The kind of applications where this advantage is important include:
o Hot water tanks
o Brewing tanks
o Process plant
o Swimming pool structures
How the Austenite/Ferrite Balance is Achieved
To understand how duplex steels work, compare the composition of two familiar steel sheets, austenitic 304 (1.4301) and ferritic 430 (1.4016).
| Structure |
Grade |
EN Number |
C |
Si |
Mn |
P |
S |
N |
Cr |
Ni |
Mo |
| Ferritic |
430 |
1.4016 |
0.08 |
1.00 |
1.00 |
0.040 |
0.015 |
– |
16.0/18.0 |
– |
– |
| Austenitic |
304 |
1.4301 |
0.07 |
1.00 |
2.00 |
0.045 |
0.015 |
0.11 |
17.5/19.5 |
8.0/10.5 |
– |
The important elements in stainless steel can be classified into fertilizers and austerities. Each element favors one structure or the other, as follows:
Ferritisers – Cr (chromium), Si (silicon), Mo (molybdenum), W (tungsten), Ti (titanium), Nb (niobium)
Austenitisers – C (carbon), Ni (nickel), Mn (manganese), N (nitrogen), Cu (copper)
Grade 430 predominates fertilizers, and so is ferritic in structure. Grade 304 becomes austenitic mainly through the use of about 8% nickel. To arrive at a duplex structure with about 50% of each phase, there has to be a balance between the austerities and the fertilizers. This explains why the nickel content of duplex steels is generally lower than that of austenitic.
Here are some typical compositions of duplex stainless steels:
| Grade |
EN No/UNS |
Type |
Approx. Composition
|
| |
|
|
Cr |
Ni |
Mo |
N |
Mn |
W |
Cu |
| 2101 LDX |
1.4162/S32101 |
Lean |
21.5 |
1.5 |
0.3 |
0.22 |
5 |
– |
– |
| DX2202 |
1.4062/S32202 |
Lean |
23 |
2.5 |
0.3 |
0.2 |
1.5 |
– |
– |
| RDN 903 |
1.4482/S32001 |
Lean |
20 |
1.8 |
0.2 |
0.11 |
4.2 |
– |
– |
| 2304 |
1.4362/S32304 |
Lean |
23 |
4.8 |
0.3 |
0.10 |
– |
– |
– |
| 2205 |
1.4462/S31803/S32205 |
Standard |
22 |
5.7 |
3.1 |
0.17 |
– |
– |
– |
| 2507 |
1.4410/S32750 |
Super |
25 |
7 |
4 |
0.27 |
– |
– |
– |
| Zeron 100 |
1.4501/S32760 |
Super |
25 |
7 |
3.2 |
0.25 |
– |
0.7 |
0.7 |
Ferrinox
255/
Uranus 2507Cu |
1.4507/S32520/S32550 |
Super |
25 |
6.5 |
3.5 |
0.25 |
– |
– |
1.5 |
In some recently developed grades, nitrogen and manganese are used to bring the nickel content to very low levels, which benefits price stability.
At present, we are still very much in the development phase of duplex steels. Therefore, each mill is promoting its own particular brand. It is generally agreed that there are too many grades. However, this is likely to continue until the “winners” emerge.
Corrosion Resistance of Duplex Steels
The range of duplex steels allows them to be matched for corrosion resistance with the austenitic and ferritic steel grades. There is no single measure of corrosion resistance. However, it is convenient to use the Pitting Resistance Equivalent Number (PREN) as a means of ranking the grades; one of the commonly used formulae for this parameter is:
PREN = %Cr + 3.3 x %Mo + 16 x %N
The following table shows how the duplex steels compare with some austenitic and ferritic grades.
| Grade |
EN No/UNS |
Type |
Typical PREN |
| 430 |
1.4016/S43000 |
Ferritic |
18 |
| 304 |
1.4301/S30400 |
Austenitic |
19 |
| 441 |
1.4509/S43932 |
Ferritic |
19 |
| RDN 903 |
1.4482/S32001 |
Duplex |
22 |
| 316 |
1.4401/S31600 |
Austenitic |
24 |
| 444 |
1.4521/S44400 |
Ferritic |
24
|
| 316L 2.5 Mo |
1.4435 |
Austenitic |
26 |
| 2101 LDX |
1.4162/S32101 |
Duplex |
26 |
| 2304 |
1.4362/S32304 |
Duplex |
26 |
| DX2202 |
1.4062/ S32202 |
Duplex |
27
|
| 904L |
1.4539/N08904 |
Austenitic |
34 |
| 2205 |
1.4462/S31803/S32205 |
Duplex |
35 |
| Zeron 100 |
1.4501/S32760 |
Duplex |
41 |
Ferrinox 255/
Uranus 2507Cu |
1.4507/S32520/S32550 |
Duplex |
41 |
| 2507 |
1.4410/S32750 |
Duplex |
43 |
| 6% Mo |
1.4547/S31254 |
Austenitic |
44 |
It must be emphasized that this table is only a guide to material selection. It is always important to assess the suitability of a particular with a full knowledge of the corrosive environment.
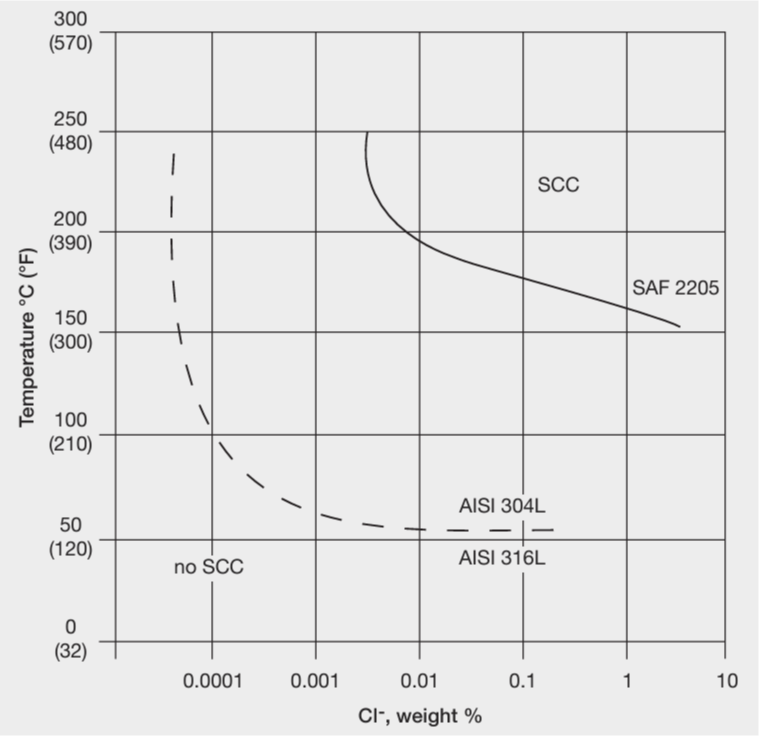
Stress Corrosion Cracking (SCC)
SCC is a form of corrosion which occurs with a particular combination of factors:
- Tensile stress
- Corrosive environment
- Sufficiently high temperature. Normally, it is 50 deg. C, but it can occur at lower temperatures, around 25 deg. C, in specific environments, notably swimming pools.
Unfortunately, standard austenitic steels like 304 (1.4301) and 316 (1.4401) are the most susceptible to SCC. The following materials are much less prone to SCC:
- Ferritic stainless steels
- Duplex stainless steels
- High nickel austenitic stainless steel
The resistance to SCC makes duplex steels suitable materials for many processes that operate at higher temperatures, notably:
- Hot water boilers
- Brewing tanks
- Desalination
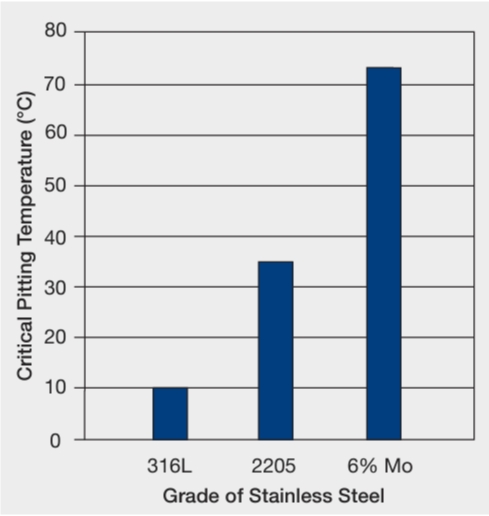
Stainless steel structures in swimming pools are known to be prone to SCC. Standard austenitic stainless steels like 304 and 316 are forbidden in this application, i.e., where there are load-bearing requirements and/or safety considerations. The best steels for this purpose are the high nickel austenitic steels, such as the 6% Mo grades. However, duplex steels such as 2205 (1.4462) and the super duplex grades can be considered in some cases.
Barriers to Using Duplex Steels
The attractive combination of high strength, a wide range of corrosion resistance, and moderate weldability would seem to offer great potential for increasing the market share of duplex stainless steel. However, it is important to understand their limitations and why they will always likely be “niche players.”
The advantage of high strength immediately becomes a disadvantage when considering formability and machinability. High strength also comes with lower ductility than austenitic grades. Therefore, any application requiring a high degree of formability, such as a sink, is ruled out for duplex grades. Even when the ductility is adequate, higher forces are required to form the material, such as tube bending. One exception to the normal rule of poorer machinability is grade 1.4162.
The metallurgy of duplex stainless steels is much more complex than that of austenitic or ferritic steels. This is why three-day conferences can be devoted just to duplexes! This factor also means they are more challenging to produce and fabricate at the mill.
In addition to ferrite and austenite, duplex steels can form several unwanted phases if the steel is not properly processed, notably in heat treatment. Two of the most important phases are illustrated in the diagram below:
| Sigma phase |
 |
| 475-degree embrittlement |
Both phases lead to embrittlement, i.e., loss of impact toughness.
Sigma phase formation is likely when the cooling rate during manufacture or welding is not fast enough. The more highly alloyed the steel, the higher the probability of sigma phase formation. Therefore, super duplex steels are most prone to this problem.
475-degree embrittlement is due to the formation of a phase called α′ (alpha prime). Although the worst temperature is 475 deg. C, it can still form at temperatures as low as 300 deg. C. This leads to a limitation on the maximum service temperature for duplex steels. This restriction reduces the potential range of applications even further.
At the other end of the scale, there is a restriction on the low-temperature use of duplex stainless steels compared to austenitic grades. Unlike austenitic steels, duplex steels exhibit a ductile-brittle transition in the impact test. A typical test temperature is minus 46 deg. C for offshore oil and gas applications. Minus 80 deg. C is the lowest temperature that is usually encountered for duplex steels.
Summary of Duplex Characteristics
- Twice design strength of austenitic and ferritic stainless steels
- Wide range of corrosion resistance to match application
- Good toughness down to minus 80 deg. C, but not genuine cryogenic applications
- Particular resistance to stress corrosion cracking
- Weldable with care in thick sections
- More difficult to form and machine than austenitic
- Restricted to 300 deg. C maximum
5. Precipitation hardening (PH) – These steels can develop high strength by adding Copper, Niobium, and Aluminium to the steel. With a suitable “aging” heat treatment, excellent particles form in the steel matrix, which imparts strength. These steels can be machined to quite intricate shapes, requiring good tolerances before the final aging treatment, as there is minimal distortion from the final treatment. This contrasts conventional hardening and tempering in martensitic steels, where distortion is more of a problem. Corrosion resistance is comparable to standard austenitic steels like 1.4301 (304).
PRECIPITATION HARDENING STAINLESS STEELS
Introduction
Stainless steel is the name given to a family of corrosion and heat-resistant steels containing a minimum of 10.5% chromium. Just as there is a range of structural and engineering carbon steels meeting different requirements of strength, weldability, and toughness, so there is a wide range of stainless steels with progressively higher corrosion resistance and strength levels. This results from the controlled addition of alloying elements, each offering specific attributes regarding strength and ability to resist different environments. The available grades of stainless steel can be classified into five basic families: ferritic, martensitic, austenitic, duplex, and precipitation hardening.
Precipitation Hardening Stainless Steels
Precipitation hardened, PH, stainless steels are a class of stainless steels that can be hardened to significant strength levels by special heat treatments. These alloys were first introduced in the mid-1940s to fulfill the requirement for high-strength corrosion-resistant alloys with higher toughness than the plain martensitic stainless steels (and increased tempering-off/softening resistance when exposed to slightly elevated temperatures). Many different alloys have been developed over the subsequent years, and they are now widely used in aerospace, marine, automotive, and other specialist applications. These alloys are used whenever a combination of high strength, corrosion resistance, and toughness is required, which no other type of stainless steel can provide. Precipitation hardening is typically achieved by adding elements such as copper, molybdenum, niobium, aluminum, and titanium in various combinations and levels. After heat treatment, they can give tensile strengths ranging from 850 MPa. to 1700 MPa. and yield strengths ranging from 520 MPa. to over 1500 MPa. Thus, they are about three to four times stronger than the common austenitic stainless steels such as Type 304L, BS EN 1.4307, and Type 316L, BS EN 1.4404.
The family of precipitation-hardening stainless steels comprises three main sub-groups describing their matrix structure, i.e., Martensitic, Semi-Austenitic, and Austenitic. They are united by the fact that they use a precipitation/aging mechanism to achieve their hardness. One can look at these stainless steels as thus containing alloying elements that control their final matrix structure (i.e., mainly the chromium and nickel balance) and other alloying elements (as already indicated), which form precipitate in that matrix and harden it. In some cases, these age-hardening elements, as they precipitate out and/or cluster during the pre-precipitation stage, can also alter the stability of the matrix phases and, thus, the final matrix phase balance.
- Martensitic Precipitation Hardening Stainless Steels
Historically, these were the first PH steels to be developed, and a typical example of this group is 17-4 PH, BS EN 1.4542, UNS S17400. As can be seen in the summary table below, the “17-4” refers to the steel’s approximate chromium and nickel contents, respectively. For the precipitation/age-hardening effect, the steel is alloyed with copper and niobium. During the hardening cycle, it transforms to martensite at low temperatures, typically around 250°C and is further strengthened by aging at about 482°C. The most common product form is a bar, but there is some availability as castings, sheets, or plates, particularly in the USA, where these steels were first developed. Cold forming of these alloys is difficult because the hard, untempered martensitic structure developed after the solution heat treatment step. Alloys in this condition have relatively low ductility and high strength. Hardening by a single aging treatment will produce tensile strengths typically from 790 MPa to 1310 MPa. This alloy can be used at temperatures up to around 470°C, i.e., below the aging temperature. Where the original aging reaction is produced at higher temperatures in other steels in this sub-group, the temperatures encountered in its application can be correspondingly higher.
As shipped from the mill, the material will usually be in the solution heat-treated condition (Condition A), thus ready for fabrication and finally hardening by the customer. However, it can be supplied hardened or in an overaged condition for forging/cold heading at the customer’s request.
Thus, the possible as-supplied conditions are as follows:-
-
- Condition A (Solution Treated/Annealed) is used when the user will carry out fabrication and precipitation heat treatment. However, if severe cold forming is needed, then Condition H 1150 or H 1150-M is suggested
- Condition H 1075 Precipitation hardened condition, machinability is similar to Condition A’s.
- Condition H 1150 Precipitation hardened condition. Fabrication is easier than material in Condition A. Providing subsequent deformation is insignificant; no further heat treatment steps are necessary.
- Condition H 1150-M This has a softer martensite matrix, thus improving the steel’s machinability.
- Condition H 1150 + H1150 This is a heat treatment meeting NACE MR01750/ISO 1516 and NACE MR0103; it is sometimes referred to as H 1150-D, where D means Double precipitation hardening.
- Semi-Austenitic Precipitation Hardening Stainless Steels
As one might expect from their name, this sub-group has a mixed matrix microstructure consisting of austenite and martensite. The typical example of this group is 17-7 PH, BS EN 1.4568, UNS S17700, and again, its commonly used name, “17-7”, indicates its chromium and nickel contents, respectively. The alloying element added to give the age-hardening effect in this particular alloy is aluminum, as seen in the table below. As supplied from the mill, usually, this grade will have been solution treated to Condition ‘A’ by heating to approximately 1066℃ (1950°F) and holding for up to 4 hours. The user then fabricates the component required from the condition ‘A’ material. At this point, there are three main routes that the processing can follow:-
-
- Route 1. This is where the steel was heavily cold-worked during fabrication (called Condition ‘C’). The steel is then heated to 482°C (900°F), held for at least 1 hour, and air-cooled. The material is now in condition CH 900. It is generally acknowledged that this gives the highest aged strength levels, as per AMS 5529.
- Route 2. The first step is the austenite conditioning step, where the steel is heated to 955°C (1750°F), held for approximately 10 minutes, and then air cooled. The material is now said to be in condition ‘A1750’.
Within a maximum of 1 hour, the steel is cooled to -73°C (-100°F) and held at that temperature for 8 hours, after which it is allowed to warm in the air back to room temperature. The material is now said to be in condition ‘R100’. This is called the transformation step.
In the final stage, the steel is heated to 510°C (950°F), held for 90 minutes, then air-cooled to room temperature. The steel is now said to be in condition ‘RH950’. This precipitation hardening/aging step results in a material with intermediate final strength levels.
- Route 3. Again, the first step is austenite conditioning, but the steel is heated to a lower temperature of 760°C (1400°F), held for approximately 90 minutes, and then within 1 hour cooled to 13°C (55°F), held for at least 30 minutes. The material is now said to be in condition ‘T.’ This latter step is again called the transformation step.
In the final step, the steel is heated to 565°C (1050°F), held for 90 minutes, and then air cooled to room temperature. The steel is now in condition ‘TH1050’—this precipitation hardening/aging step results in this alloy’s lowest final aged strength levels.
This somewhat complicated list of possible processing options is typical of this precipitation-hardening stainless steel subgroup.
Rapid cooling from the mill solution treatment temperature to room temperature retains a fully austenitic structure; this provides these steels with the necessary ductility for cold-forming processes, unlike the martensitic PH steels, which tend to be excessively hard at the same stage. An initial transformation from austenite to martensite is necessary to induce hardening and strengthening. This prepares the material for subsequent treatment at the aging temperature. Heating semi-austenitic PH steels to the range of 650oC–870°C prompts the precipitation of carbides. As alluded to above, this lowers the level of the alloying elements in the matrix, particularly those that stabilize austenite, thus allowing a degree of transformation to martensite upon subsequent cooling to room temperature. Partial martensite transformation of the austenite can also be achieved by refrigeration below the Ms temperature (the beginning of martensite transformation) or through cold working (the Md temperature being higher than the Ms temperature).
The other effect that is apparent with this sub-group is that because the solubility of the alloying elements increases at higher temperatures, the martensite start, Ms, and finish Mf temperatures can be controlled by the solution heat-treatment temperatures. At high solution temperatures, the alloy content of austenite increases, and the martensite start temperature is depressed. At lower solution temperatures, the austenite is leaner in alloy content (i.e., lower levels are in solution) and, upon cooling, transforms to martensite.
Whatever the route, the final step is always an aging step, which hardens the steel. The hardening does not come primarily from dispersion strengthening but rather because the precipitates exhibit varying degrees of coherency of their crystal lattice with that of the parent structure. A mismatch between the two lattices produces strain in the parent lattice and, thus, a hardening effect. The material can be overaged, and in this stage, the precipitates start to lose coherency with the matrix, and their hardening effect diminishes. As well as the kinetics of aging, the aging temperature will affect the number of precipitates per unit volume, with a more numerous finer dispersion resulting from lower aging temperature, but precipitation will be slower.
The best availability of sheet and strip products in the USA is for grades PH 15-7 Mo and AM-350, whereas in the UK, 17-7 PH and FV520, BS EN 1.4594, and UNS S45000 have the best availability. Grade 15-7 PH is similar to the 17-7 PH alloy but has a low molybdenum content (see table below), providing even higher strength levels in the age-hardening process. AM-350 is similar to 15-7PH and 17-7 PH but has a slightly better high-temperature capability. FV520B employs molybdenum, copper, and niobium as the precipitate-forming elements and is a “14-5” PH steel, as seen in the summary table below.
For some of these alloys, a refrigeration step (e.g., -50oC/-60oC for eight hours) is always necessary to transform them to a stable austenitic/martensitic structure, although the two most commonly used alloys, FV520 and 17/7PH, do not require refrigeration to develop optimum properties. (*However, the optimum in one application may not necessarily be the optimum combination of properties for another application.)
- Austenitic Precipitation Hardening Stainless Steels
These alloys have a fully austenitic matrix, so their hardness comes from the precipitates that form on aging. The most common alloy in this subgroup is A286; sources also cite 17-10 P, but this is not a popular alloy with very limited availability. For precipitation, the former is alloyed with molybdenum, aluminum, titanium, vanadium, and boron. It would be referred to as “15-26” PH, using the system for referring to the other PH alloys. In 17-10 P, phosphorus is the precipitate-forming element; see summary table below, which is suggested to affect its weldability adversely.
Austenitic alloys maintain their austenitic structures through annealing and subsequent hardening via aging. At the annealing temperature of 1095oC –1120oC, the precipitation hardening phase dissolves and remains in solution during rapid cooling.
These alloys age at temperatures between 500°C and 760°C. The austenitic grades are stable down to room temperature, and improvements in strength are from the precipitates formed by aging at 650°C to 750°C. These fully austenitic grades can exhibit good toughness; some may be used at cryogenic temperatures.
Austenitic alloys’ strength and hardness are lower than martensitic or semi-austenitic PH grades and retain their non-magnetic properties.
Nominal Chemical Composition of Selected PH Stainless Steels
| Alloy |
UNS No. |
Typical Composition, % |
| C |
Mn |
Si |
Cr |
Ni |
Mo |
Cu |
Ti |
Other |
| Martensitic |
| PH 13-8Mo |
S13800 |
0.05 |
0.10 |
0.10 |
12.80 |
8.00 |
2.30 |
|
|
Al=1.1 |
| 15-5 PH |
S15500 |
0.07 |
1.00 |
1.00 |
14.80 |
4.50 |
– |
3.50 |
|
Nb=0.3 |
| 17-4 PH |
S17400 |
0.09 |
1.00 |
1.00 |
16.30 |
4.00 |
– |
4.00 |
|
Nb=0.3 |
| Custom 455 |
S45500 |
0.05 |
0.50 |
0.50 |
12.00 |
8.50 |
0.50 |
2.00 |
1.10 |
Nb=0.3 |
| Semi-austenitic |
| 15-7Mo PH |
S15700 |
0.09 |
1.00 |
1.00 |
15.00 |
7.10 |
2.50 |
– |
– |
Al=1.1 |
| 17-7 PH |
S17700 |
0.08 |
0.90 |
0.50 |
16.50 |
7.50 |
– |
|
|
Al=1.0 |
| AM-350 |
S35000 |
0.09 |
0.80 |
0.30 |
16.50 |
4.30 |
2.75 |
|
|
N=0.10 |
| FV 520 |
S45000 |
0.05 |
0.60 |
|
14.50 |
4.75 |
1.40 |
1.70 |
|
Nb=0.3 |
| Sandvik Nanoflex |
S46910 |
<0.012 |
|
|
12.00 |
9.00 |
4.00 |
2.00 |
0.90 |
Al=0.35 |
| Austenitic |
| A-286 |
S66286 |
0.08 |
2.00 |
1.00 |
15.00 |
25.50 |
1.25 |
– |
– |
Ti=2.1, Al</=0.35, V=0.3 |
| 17-10P PH |
|
0.07 |
0.75 |
|
17.20 |
10.80 |
|
|
|
P=0.28 |
Comparison Table of the Main Characteristics of Different Types of Stainless Steel
| Stainless Steel Types |
Magnetic Response |
Work Hardening Rate |
Corrosion Resistance |
Hardenable |
Ductility |
High Temperature Resistance |
Low Temperature Resistance |
Weldability |
| Austenitic |
Generally No |
Very High |
High |
By Cold Work |
Very High |
Very High |
Very High |
Very High |
| Duplex |
Yes |
Medium |
Very High |
No |
Medium |
Low |
Medium |
High |
| Ferritic |
Yes |
Medium |
Medium |
No |
Medium |
High |
Low |
Low |
| Martensitic |
Yes |
Medium |
Medium |
Quench & Temper |
Low |
Low |
Low |
Low |
| Precipitation Hardening |
Yes |
Medium |
Medium |
Age Harden |
Medium |
Low |
Low |
High |
Conclusion
Understanding the different types of stainless steel is crucial for selecting the right material for your needs. The five main categories—Austenitic, Ferritic, Martensitic, Duplex, and Precipitation-Hardening stainless steels—each offer unique properties that make them suitable for various applications. From excellent corrosion resistance and formability in austenitic steels to the high strength and toughness of duplex steels, knowing these distinctions helps ensure your projects’ optimal performance and longevity. By leveraging the strengths of each stainless steel type, industries can achieve greater efficiency, safety, and durability in their products and structures. Whether you are in the aerospace, automotive, medical, or construction industry, selecting the appropriate stainless steel type is essential for success.