Nickel and its applications
Nickel Applications
Nickel (Ni), a chemical element, and ferromagnetic metal of Group 10 (VIIIb) of the periodic table, is markedly resistant to oxidation and corrosion. It is Silvery white, tough, and harder than iron, nickel is widely familiar because of its use in coinage but is more important either as the pure metal or in the form of alloys for its many domestic and industrial applications. Elemental nickel very sparingly occurs together with iron in terrestrial and meteoric deposits.
| Element Properties | |
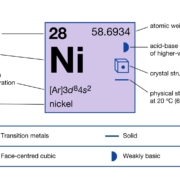
| atomic number | 28 |
| atomic weight | 58.69 |
| melting point | 1,453 °C (2,647 °F) |
| boiling point | 2,732 °C (4,950 °F) |
| density | 8.902 (25 °C) |
| oxidation states | 0, +1, +2, +3 |
| electron configuration | [Ar]3d84s2 |
Nickel will alloy readily with many other metals, including chromium, iron, molybdenum and copper. This allows for a wide variety of alloys that demonstrate outstanding resistance to corrosion and high-temperature scaling, exceptional high-temperature strength and other unique properties, such as shape memory and low coefficient of expansion.
The following is a simplistic categorization of the various nickel alloy types.
Wrought Nickel
Pure nickel UNS N02200 is used in the chemical industry for its corrosion resistance – particularly to alkalis. It is also used for its properties in shielding against electromagnetic interference and in transducers.
Nickel-Iron Alloys
These are used as soft magnetic materials, as glass-to-metal seals and as materials with defined thermal expansion properties.
Invar® (UNS K93600), with 36% nickel and the remainder iron, is unique in having an almost zero coefficient of thermal expansion around room temperature. This makes it valuable where high dimensional stability is required, such as in precision measuring instruments and thermostat rods. It is also used at cryogenic temperatures because of its very low thermal expansion rates.
Alloys containing 72-83% nickel have the best soft magnetic properties and are used in transformers, inductors, magnetic amplifiers, magnetic shields and memory storage devices.
Nickel-Copper Alloys
These are highly resistant to corrosion by alkaline solutions, non-oxidizing salts and seawater. The best-known is Alloy 400.
Nickel-Molybdenum Alloys
These are highly resistant to reducing acids in the absence of oxidizing ions, such as ferric and cupric or dissolved oxygen. The best-known is Alloy B-2.
Nickel-Chromium Alloys
These are characterized by their high resistance to corrosion at both normal and high temperatures (resistance to scaling), good high-temperature strength and high electrical resistance. There are three main groups of alloys:
Ni-Cr (and also Ni-Cr-Fe) alloys with high electrical resistance for heating elements, such as 70-30 (UNS N06008) and C-Grade (UNS N06004)
Ni-Cr alloys (with Fe and other alloying elements) with good corrosion resistance. The best-known are Alloy 600 (UNS N06600) and Alloy 601 (UNS N06601)
Ni-Cr alloys with high-temperature strength and creep resistance, mostly age-hardenable, such as Alloy X-750 (UNS N07750)
Nickel-Chromium-Iron Alloys
There are basically two groups of alloys:
Ni–Cr–Fe alloys with excellent strength at high temperatures and the ability to resist oxidation, carburization and other types of high-temperature corrosion. The best-known is alloy 800 (UNS N08800) and its variants 800H (UNS N08810) and 800HT (UNS N08811). (Recently, these alloys were classified as stainless steels reflecting their high Fe content)
Ni–Cr–Fe (with Mo and Cu) alloys with excellent corrosion resistance in specific applications. Probably the best-known is alloy 825 (UNS N08825), which offers exceptional resistance to sulphuric acid. Alloy G-3 (UNS N06985) offers exceptional corrosion resistance to commercial phosphoric acids as well as many complex solutions containing highly oxidizing acids.
Nickel-Chromium-Molybdenum Alloys
These are highly corrosion-resistant, of which Alloy C-276 (N10276) is the best-known. They offer exceptional resistance to reducing acids such as hydrochloric and sulphuric. There are a number of variants based on this composition, which have modified the Cr and Mo levels and, in some cases, added Cu or W in order to extend the corrosion resistance to conditions that are more oxidizing or more reducing. These include Alloy C-22 (N06022), Alloy 59 (N08059), Alloy C-2000 (UNS N06200), and Alloy 686 (N06686).
Nickel-Chromium-Cobalt Alloys
The addition of cobalt and molybdenum imparts solid-solution strengthening and high levels of creep-rupture strength to alloy 617 (UNS N06617). The addition of cobalt to HR-160 (N12160) provides outstanding resistance to various forms of high-temperature corrosion attacks, such as sulphidation and chloride attack in both reducing and oxidizing atmospheres.
Nickel-Titanium Alloys
55% nickel-titanium alloy (UNS N01555) (also known as Nitinol) has shape-memory properties. When formed at one temperature and then deformed at a lower one, it regains its original form when reheated. The transition temperatures can be adjusted through careful control of the composition. Medical devices and specialized connectors are two of specific the applications. The same alloy can also undergo considerable elastic deformation and still return to its original shape (super-elastic property). This property has been exploited for applications as diverse as spectacle frames and shock absorbers that provide earthquake resistance in historic stone buildings.
The nominal composition of various nickel alloys:
| Alloy name | UNS | % Ni | % Cr | % Fe | % Mo | % Cu | % Co | % Other |
| 200 | N02200 | 99 min | ||||||
| Invar | K93600 | 36 | 64 | |||||
| 400 | N04400 | 65 | 32 | |||||
| B-2 | N10665 | 68 | 2 | 28 | ||||
| 70-30 | N06008 | 70 | 30 | |||||
| C-Grade | N06006 | 60 | 16 | bal | ||||
| 600 | N06600 | 76 | 16 | 8 | ||||
| 601 | N06601 | 60 | 23 | bal | Al – 1.3 | |||
| X-750 | N07750 | 70 | 15 | 7 | Ti – 2.5, Al – 0.7, Nb – 1.0 | |||
| 825 | N08825 | 42 | 21 | 25 | 3 | 2 | ||
| G-3 | N06985 | bal | 22 | 20 | 7 | 2 | ||
| 800 | N08800 | 32 | 21 | 45 | W – 3.5 | |||
| C-276 | N10276 | bal | 15 | 5 | 16 | |||
| C-22 | N06022 | bal | 21 | 4 | 13 | |||
| 59 | N06059 | bal | 23 | 1.5 max | 16 | |||
| C-2000 | N06200 | bal | 23 | 3 max | 16 | 1.6 | ||
| 686 | N06686 | bal | 21 | 2 max | 16 | W – 3.7 | ||
| 617 | N06617 | 44.5 min | 22 | 3 max | 9 | 12 | ||
| HR-160 | N12160 | bal | 28 | 3.5 max | 30 | Si – 2.75 | ||
| Nitinol | N01555 | 55 | Ti – 45 |
| Alloy Type | UNS | %Cr | %Ni | %Mo | %Cu | %Fe | Features and Applications |
| 304L | S30403 | 18 | 8 | – | – | bal | The most common stainless steel is used for potable water treatment and food contact. |
| 316L | S31603 | 16 | 10 | 2.1 | – | bal | The addition of Mo provides greater resistance to chlorides and acidic solutions. Used in coastal regions, in water treatment and the chemical processing industry. |
| 2205 | S32205 | 22 | 5.7 | 3.1 | – | bal | Even greater corrosion resistance than 316L. Can be used in place of 316L, but its greater strength can mean weight reduction. May be substituted for 316L if it fails in service. |
| 2507 | S32750 | 25 | 7 | 4 | – | bal | Even greater corrosion resistance than 2205. Useful corrosion resistance to seawater. |
| 254 SMO | S31254 | 20 | 18 | 6.1 | – | bal | Corrosion resistance is similar to 2507, also possessing useful corrosion resistance to seawater. |
| Alloy 20 | N08020 | 20 | 33 | 2.1 | 3.2 | bal | Cu bearing stainless steel with useful corrosion resistance to all concentrations of sulphuric acid. |
| 310S | S31008 | 25 | 20 | – | – | bal | High chromium stainless steel with excellent resistance to high-temperature oxidation. |
| 800H | N08810 | 20 | 32 | – | – | bal | Stainless steel with excellent high-temperature strength and useful resistance to high-temperature oxidation. |
| 625 | N06625 | 21 | bal | 9 | – | 3 | Well-known nickel alloy with excellent high-temperature strength and outstanding aqueous corrosion resistance. |
| C-276 | N10276 | 15 | bal | 16 | – | 5 | One of the best-known nickel alloys with excellent corrosion resistance to reducing acids. |
| 600 | N06600 | 16 | 76 | – | – | 8 | Useful resistance to high-temperature corrosion and caustic solutions. |
| Alloy 400 | N04400 | – | 65 | – | 32 | – | The most common uses are in marine and chemical processing. |